electronic configuration of ti|electron configuration for au+ ion : Pilipinas To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). The video where you can see the torture and execution of a man, known as THE "gore video I want water" was the work of the hitman known as "The Clown". The victim who is now known as the Mexican Ghost Rider was an inhabitant of the municipality of Cotija in the state of Michoacán, who was kidnapped by CJNG hitmen commanded by 'El Clown', .Examples Of Spoken Poetry In Tagalog SPOKEN POETRY – In this article, we will learn more about spoken poetry, its importance, and some examples in Tagalog. Spoken word poetry is an art form that .
PH0 · titanium ground state electron configuration
PH1 · electronic configuration pdf
PH2 · electronic configuration of titanium
PH3 · electron configuration of sodium ion
PH4 · electron configuration for every element
PH5 · electron configuration for au+ ion
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa
Denise G. Sanchez and Titus Welliver, Bosch: Legacy Warrick Page/Prime Video Bosch: Legacy Season 2 release date. Freevee recently announced that Bosch: Legacy Season 2 will premiere October 20 .
electronic configuration of ti*******To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti).electronic configuration of tiThe full electron configuration of titanium is 1s2 2s2 2p6 3s2 3p6 4s2 3d2 and the abbreviated electron configuration is [Ar]3d24s2. With the Electron configuration of each .
Titanium dioxide electronic configuration. The electronic configuration of Ti in Titanium dioxide is 1s 2 2s 2 2p 2 3s 2 3p 6 3d 0 4s 0 and oxygen is found to be 1s 2 .
Titanium electron configuration. ← Electronic configurations of elements. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. .
Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and .
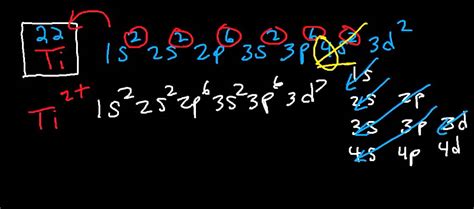
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble gas notation as well. This video.Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . Electron configuration of Titanium is [Ar] 3d2 4s2. Possible oxidation states are +2,3,4. Electron Configuration. The periodic table is a tabular display of the chemical .Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Titanium Ions) Wayne Breslyn. 6625. views. 1. rank. 03:32. A Level Chemistry Revision "Electron Configuration of Ions" Freesciencelessons. 364. .The chemical symbol of Chromium is ‘Ti’ Electronic configuration of d-block. In general, the electronic configuration of these elements is (n-1) d 1-10 ns 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost n s orbital may have one or two electrons.In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .electronic configuration of ti electron configuration for au+ ion configuration of `Ti^(3+)` ion = [Ar] `3d^(1)4s^(0)`. Complex is coloured due to the presence of an unpaired electron leading d-d transition. b) Triamminetriaquachromium (III) chlorideThis page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3. Answer link. "Ti"^ (2+): ["Ar"]3d^2 A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom. In this case, titanium, "Ti", is located in period 4, group 4 of the periodic table and has an atomic number of 22. This means that a neutral titanium atom will .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . a) Give the electronic configuration of the d-orbitals of Ti in `[Ti(H_(2)O)_(6)]^(3+)` ion in the otahedral crystal field. b) Why is this complex coloured. Explain on the basis of distribution of electrons in d-orbitals,
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble.
Click here:point_up_2:to get an answer to your question :writing_hand:the correct electronic configuration of tiz 22 atom is
To determine the electron configuration of Ti, we need to understand the ordering of the energy levels and the number of electrons in each level. The electron configuration of Ti can be represented as 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^2. This means that the first energy level (n=1) contains 2 electrons in the 1s orbital.a) Give the electronic configuration of the d-orbitals of Ti in [Ti(H2O)6] 3+ ion in an octahedral crystal field. b) Explain why this complex is coloured based on the distribution of electrons in the dorbitals. c) How does the colour change on heating [Ti(H2O)6] 3+ ion?The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .
Ti I Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 3 F 2 Ionization energy 55072.5 cm-1 (6.82812 eV) Ref. SZK90 Ti II Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 4 F 3 / 2 Ionization energy 109494 cm-1 (13.5755 eV) Ref. SC85-1 (13.5755 eV) Ref. SC85An element having electronic configuration $$ [Ar] 3d^24s^2 $$ belongs to: View Solution. Q3. Among the following series of transition metal ions, the one where all metals ions have $$ 3d^2 $$ electronic configuration is. View Solution. Q4. Select incorrect match for [M (H 2 O) 6] 2 + complex.
Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Titanium Ions) Wayne Breslyn. 6625. views. 1. rank. 03:32. A Level Chemistry Revision "Electron Configuration of Ions" Freesciencelessons. 364. views. 05:07. How to Write the Electron Configuration for Ions. Wayne Breslyn. 390. views. 04:02.
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . which makes up 95% of the Ti used worldwide. We actually use 4 million tons of TiO2 each year, a lot of it for paint and other applications that need something that is .
The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels, this added stability is enough to shift an electron from one orbital to another. In heavier elements .
Book flights from Ninoy Aquino Intl (MNL) to Mandurriao (ILO) starting at ₱1,124. Search real-time flight deals from Manila to Iloilo City on Cheapflights.com.ph.
electronic configuration of ti|electron configuration for au+ ion